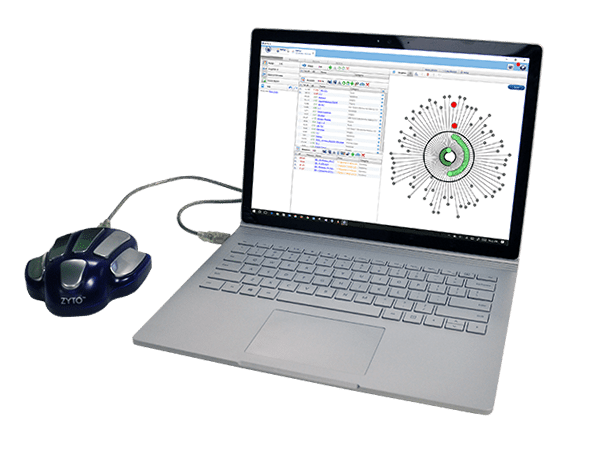
The ZYTO Hand Cradle is the industry leading hardware device cleared by the Food and Drug Administration for the measurement of Galvanic Skin Response (GSR). ZYTO’s many software offerings use the GSR data gathered by the ZYTO Hand Cradle to assist its customers in serving their clients. The Hand Cradle interfaces with the body to provide quick, accurate readings of GSR.

Based on nearly two centuries of scientific research
ZYTO Hand Cradle technology is based on established scientific principles that date back to 1849, when physician and physiologist Emil du Bois-Reymond discovered that skin was electrically active. In 1879, Dubois-Reymond’s work was advanced further when French neurologist Charles Fere found that skin resistance could change based on emotional stimulation.
Now, over 150 years later, electrodermal activity (EDA), a term now used synonymously with GSR, has been the subject of hundreds of professional publications. Those who may be skeptical that a device can accurately measure GSR need only look at the scientific studies contained in many of these publications to find that the technology is anything but pseudoscience.
Hand Cradle design maximizes accuracy
In addition to being aesthetically pleasing, the Hand Cradle is designed for maximum functionality and accuracy. The fact that it has 6 different points of contact separates it from other electrodermal and GSR devices, many of which only have 2 contact points.
Along with the advantage of gathering GSR data from multiple contacts, each contact point is also large enough to connect to several points on the skin. With more contact points covering a larger surface area, the Hand Cradle is able to provide a consistently high level of accuracy that’s unmatched in the industry.


Fast, easy GSR scanning
Establishing and maintaining a connection with the Hand Cradle is fast and easy as the finger and palm pads will typically register skin contact with just a small amount of pressure.
For those who may have trouble establishing a connection with the Hand Cradle, we’ve created a video with some simple tips to get connected.
FDA-cleared galvanic skin response device
The ZYTO Hand Cradle is the culmination of years of research and development in the field of galvanic skin response technology. It’s also the industry-leading device on the market that’s cleared by the FDA (510k Class II Medical Device).
The ZYTO Hand Cradle provides data that is, in turn, separately used by our various software products. See our Software Product Comparison page to learn more about how the GSR data gathered by the Hand Cradle is used.

Galvanic Skin Response
*By submitting this form, you also agree to receive periodic updates from ZYTO.